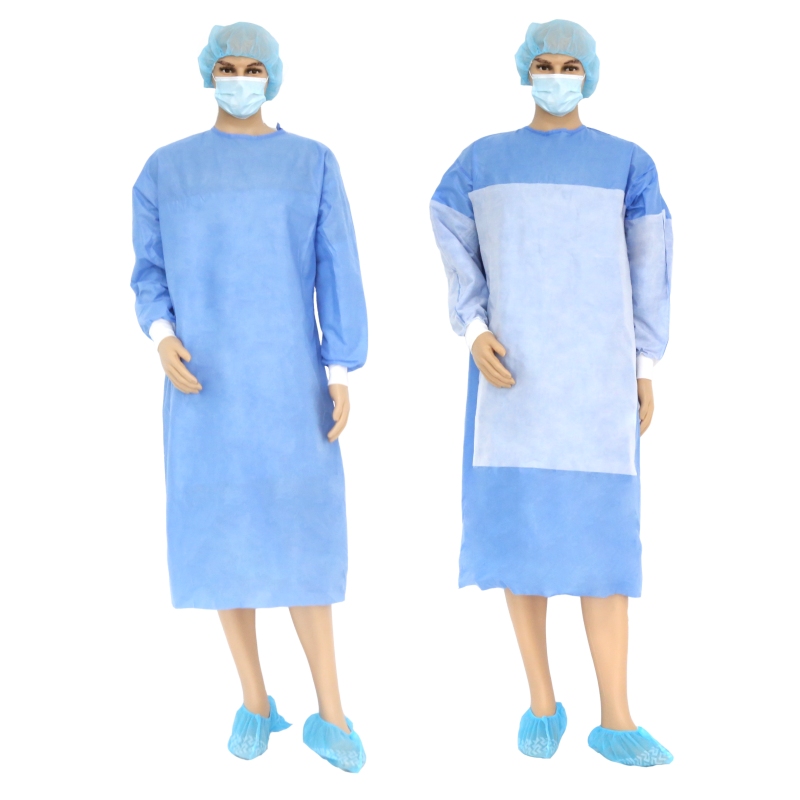
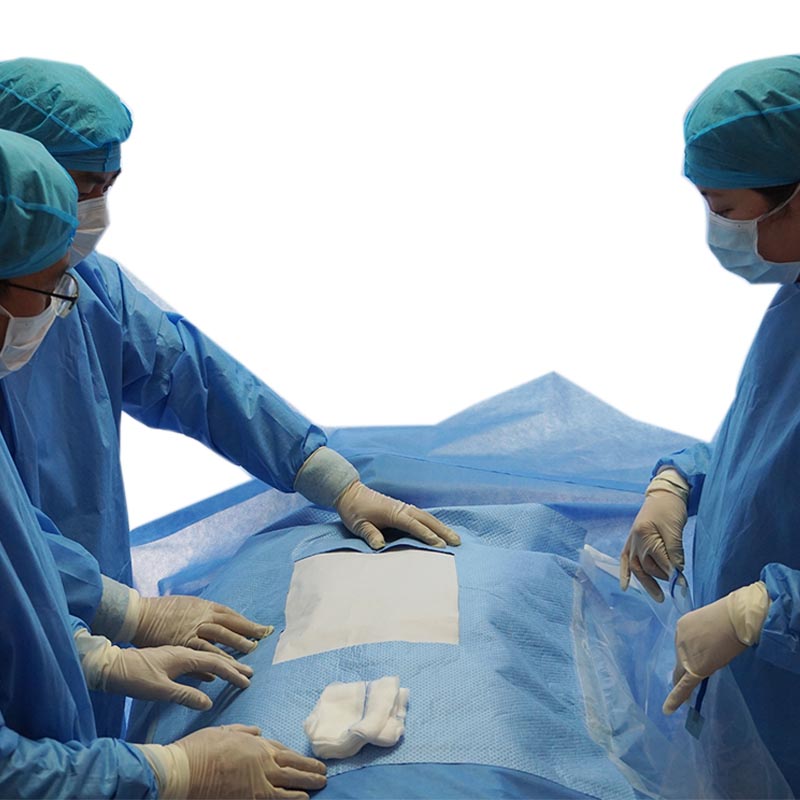
Medical isolation mask and goggles are personal protective equipment, goggles are eye protective equipment, mainly by changing the transmittance intensity and spectrum, to avoid the damage caused by radiation light to the eyes, but also can block the damage caused by the splash in the air, dust, etc...
View More
-
 WhatsAPP/Wechat +86 199 5567 1917
WhatsAPP/Wechat +86 199 5567 1917 -
 Email us info@medpurest.com
Email us info@medpurest.com










 New Products
New Products

 Leave A Mesage
Leave A Mesage





