On the evening of December 21st, the 2026 annual meeting of "Striving for 20 Years, Grateful for a New Journey" organized by kmn Group and Medpurest Medical was warmly kicked off. Family members gather, look back on the fruits of hard work in 2025, and go to a new journey full of hope with enthusias...
View More
-
 WhatsAPP/Wechat +86 199 5567 1917
WhatsAPP/Wechat +86 199 5567 1917 -
 Email us info@medpurest.com
Email us info@medpurest.com









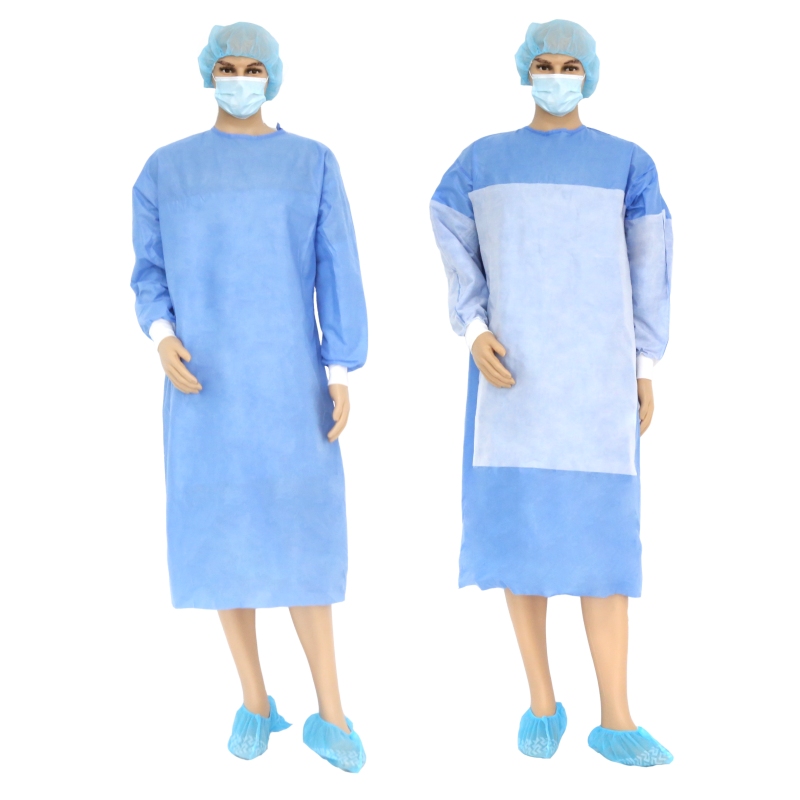
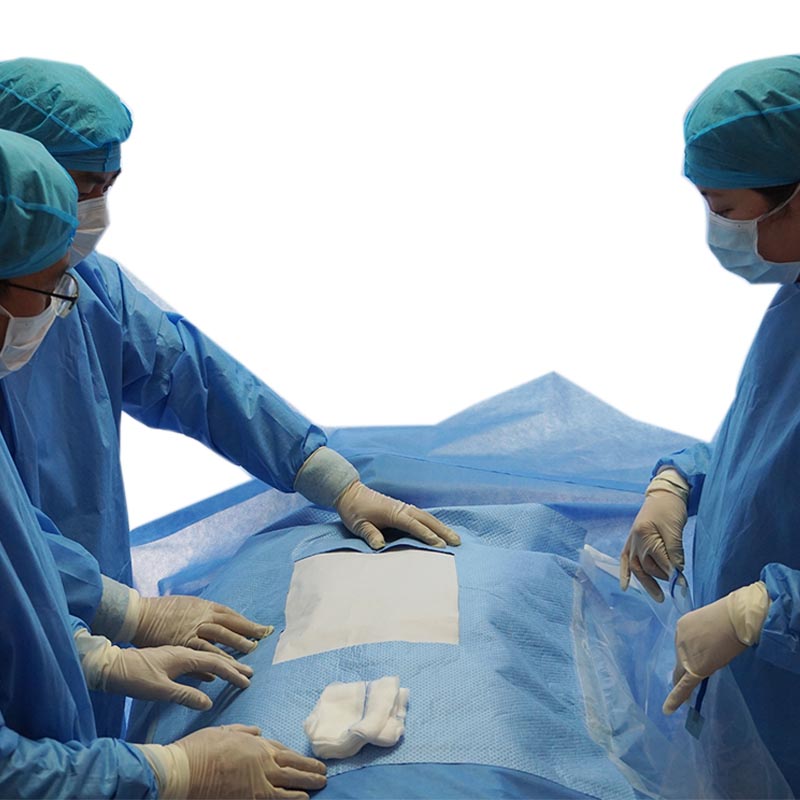
 New Products
New Products

 Leave A Mesage
Leave A Mesage





